
Quality Methods and Root Cause Analysis in Medical Technology
Master QM tools that satisfy ISO 13485 and FDA audits.

Master QM tools that satisfy ISO 13485 and FDA audits.
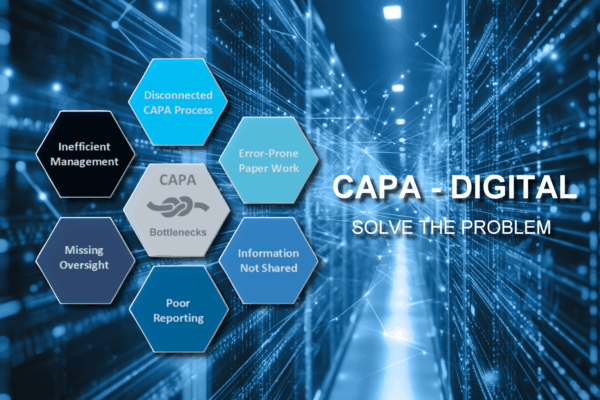
Orcanos turns CAPAs into strategic assets – get smart KPIs, insights, and audit-proof traceability.

Compliant CAPA docs made easy – ISO 13485 tips, pitfalls, and expert guidance.
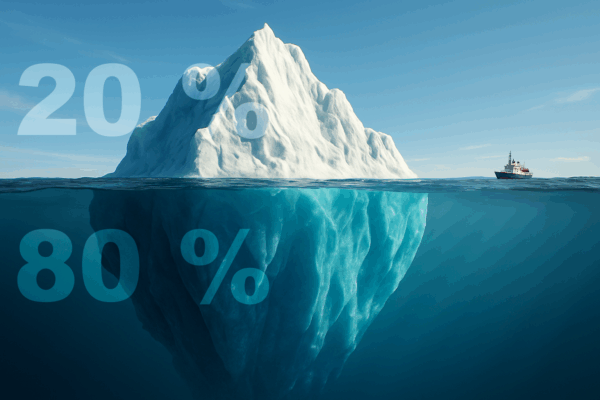
Discover how 80% of your project outcomes can be achieved with 20% of the effort. Our blog shows how engineers & managers in MedTech can apply this powerful rule.
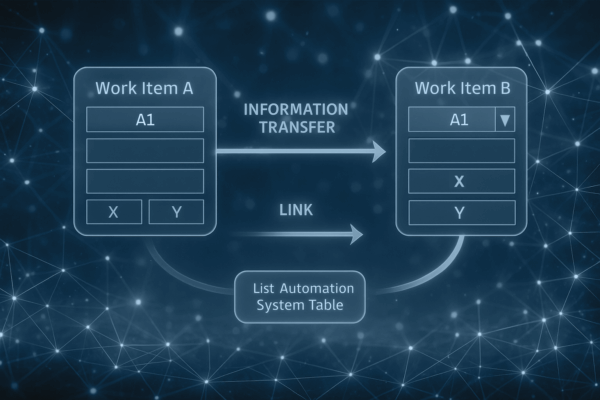
Optimize MedTech documentation with Orcanos: Reduce redundancy, improve traceability, automate data. Save time, boost quality, simplify audits!

KPIs in MedTech (ISO 13485): Optimize processes, ensure compliance & boost performance. Real examples & tools included!
+49 331 600 87 640