Introduction
Corrective and Preventive Actions (CAPAs) are a central element of quality management. Especially in regulated industries such as medical technology. They are used to systematically analyze and eliminate non-conformities and prevent future errors. CAPA requirements are described in detail in the ISO 13485:2016 standard, among others.
In a previous blog post, we explained what CAPAs mean in theoretical and regulatory contexts. In this article, we will focus on concrete implementation. How can a CAPA strategy be implemented digitally, comprehensively, and efficiently within a system like Orcanos?
As an integrated quality management system (QMS) and application lifecycle management (ALM) platform, Orcanos offers comprehensive options for the structured management and evaluation of CAPAs. Below, we explain how CAPAs can be modeled, processed, evaluated, and linked to other quality-relevant processes, such as ECO (Engineering Change Order) management, in Orcanos.
Why map CAPAs in a database like Orcanos?
CAPAs are often processed using tables or simple workflow systems. However, these methods quickly reach their limits when managing multiple, complex, or extensive CAPAs simultaneously, tracking responsibilities, or documenting correlations. This is precisely where database-supported systems, such as Orcanos, come in.
ISO 13485:2016 requires the documentation of traceable CAPA measures, their causes, checks, effectiveness, and any escalations. Orcanos fulfills these requirements with structured items that can be logically linked and evaluated. This improves the quality of documentation and makes day-to-day work easier.
One concrete advantage is the ability to link each CAPA measure to a responsible person, set a deadline, document progress, and present all relevant relationships between tasks in a simple, clear manner. This reduces queries, facilitates teamwork, and establishes a reliable database for audits.
Additionally, similarities between CAPAs can be identified. For instance, if there are multiple customer complaints about a specific product, they can be grouped together and analyzed using filter mechanisms. Thus, structured recording enables not only the processing of individual CAPAs, but also proactive quality management.
The base in Orcanos
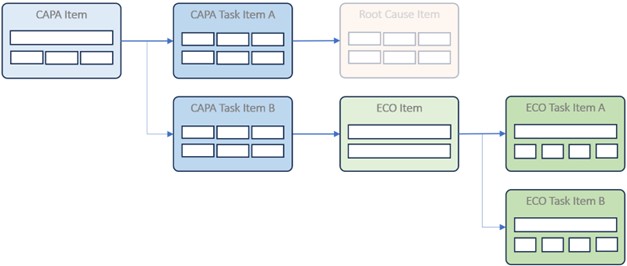
Orcanos offers a wide range of technical options for managing CAPAs in a structured, automated way. These include: system tables, work item list automation, advanced automation rules, calculated fields, and filters. The CAPA model is based on CAPA items, CAPA tasks, ECO items, ECO tasks, and other object-specific items, such as root cause items (see illustration). These items can be configured individually, depending on the actual process.
Custom fields are central to this process. As previously mentioned, these fields can be tailored to specific processes. For instance, fields such as “Process affected,” “Error description,” “Batch affected,” and “Country of origin of complaint” can be systematically maintained and evaluated later.
Advanced Automation Rules are used for additional automation. These rules allow for the automatic transfer of results from CAPA tasks to the corresponding CAPA item. This reduces manual work steps, prevents transfer errors, and ensures consistency. Further information on Advanced Automation Rules can be found in this blog post: Redundancy out, efficiency in: Making documentation smarter with Orcanos – tecurat
One particular advantage is traceability. All items can be linked using system logic. For example, a customer complaint can be linked to a CAPA, which can be linked to an ECO. This traceability is required by standards and is extremely valuable for understanding quality contexts.
The CAPA process in Orcanos - step by step
The implementation of the CAPA process in Orcanos follows a clear, structured logic. This allows both simple CAPAs and complex, cross-departmental processes to be mapped. The following figure provides an overview of the CAPA process structure.
The entry point is the CAPA item. It describes the non-conformity and identifies the fundamental problem. The central function of this item is to compile all subsequent steps and present them in an overview, including the analysis, planning, implementation, and review of CAPA measures.
CAPA tasks are created for operational processing. These tasks relate to specific aspects of CAPA. A typical CAPA consists of the following task blocks:
- Root Cause Analysis: Identify the cause of the error using structured methods such as 5 Whys, Ishikawa, or FMEA. Further methods can be found in this blog post: “Methods”
- Risk Assessment: Evaluation of which measures are necessary to prevent nonconformity from occurring again. Criteria include the severity of the problem, frequency/occurrence rate, systemic nature, and potential impact on safety and conformity. The decision is made here as to whether the deviation is transferred to the CAPA process.
- Action planning: Definition of immediate, containment, corrective, and preventive actions.
- Verification: Checking whether the measures have been implemented correctly and whether the measures have caused any additional non-conformities.
- Effectiveness: Evaluation of whether the measures contribute to sustainable error prevention.
Each of these tasks is recorded via its own CAPA task item. The person responsible, deadlines, effort estimates, and status can be assigned to each item. The link to the CAPA item ensures that information is collected centrally and merged automatically later.
Linking with ECOs - making changes traceable
In many cases, CAPAs result in changes to documents, processes, or products. For example, a work instruction may need to be adapted, or a test system may need to be modified. These changes must be planned, implemented, and documented in a traceable manner.
For this purpose, the ECO item is created in Orcanos. An Engineering Change Order (ECO) provides a structured way to manage targeted changes. Links at the task level connect the ECO item to the original CAPA process (see figure).
Example: As part of a root cause analysis, it was recognized that an inaccurate test step in the production line led to a non-conformity. The corresponding CAPA task proposes a change to the test instruction. This results in an ECO with its own tasks, such as “update document,” “obtain QA approval,” or “carry out training.”
Thanks to bidirectional linking, it is always possible to trace which CAPA led to the ECO and which measures resulted from it. This traceability fulfills ISO 13485 requirements and provides valuable support during audits.
Visualization & evaluation: Dashboards in Orcanos
One advantage of database-based CAPA systems is the ability to analyze collected information. Orcanos provides powerful, interactive dashboard functions for this purpose, enabling managers to maintain an overview at all times.
Visualization options range from simple task status overviews to complex KPI analyses. Examples of frequently used evaluations include:
- Number of open, overdue, and completed CAPAs per month
- Distribution of CAPA causes (e.g., human error, machine error, method error, material error).
- Average processing time by CAPA type or department
- Heat maps of complaint accumulations by region or product line
- The proportion of CAPAs with follow-up ECOs
This information is all based on structured custom fields and can be filtered and grouped according to individual criteria. This transforms error cases into usable data for strategic improvements.
Conclusion
Orcanos’ structured implementation of CAPAs makes quality processes standard-compliant, efficient, and transparent. Rather than relying on manual processes or isolated solutions, Orcanos offers a standardized platform that meets technical and regulatory requirements.
However, the necessary preparatory work should not be underestimated. You need a solid understanding of your processes, clear data models, and clean structures. However, the long-term benefits are significant!
A key feature is linking all relevant objects, including non-conformity, root cause analysis, ECOs, and effectiveness checks. Every step can be traced and evaluated logically. Dashboards provide real-time insights into open tasks, critical trends, and KPI developments. They enable truly measurable processes and can be used for management reviews, for example.
Automated rules, such as Advanced Automation Rules, enable seamless, automatic documentation that makes influences and correlations recognizable. Redundancies are also reduced. This enables you to pass audits with confidence. It also fosters a culture of continuous improvement.
Companies that use Orcanos as a central CAPA management tool establish a solid foundation for sustainable quality. They benefit twice over: from reliable data in day-to-day operations and valuable insights for strategic decisions.